Department of Computer Science
Programming the Swarm

|
Sponsored by the National Science Foundation |
evans@cs.virginia.edu
Selvin George
selvin@cs.virginia.edu
Cells can diffuse chemicals in the environment. These diffused chemicals follow an exponential decay and the concentration of the diffused chemical reduces quickly as the distance from the cell increases. In the present cell model, the chemical concentration at any point is written as:
conc = ( Initial Concentration - ( ( x - x0 )2 + ( y - y0 )2 + ( z - z0 )2)1/2 )
The actual concentration is econc. When programming, the exponential term is omitted and instead only the index is listed. Thus, if the initial concentration is e3, then at a distance of 1 the concentration is e2 and at a distance of 4 or more the concentration becomes almost 0. This is done because cells cannot sense very precise changes in chemicals. Thus e2 + e3 ~= e3. In effect, the diffusion model adds chemicals of different concentrations by keeping only the highest concentration. This gives a simple arithmetic model for programming
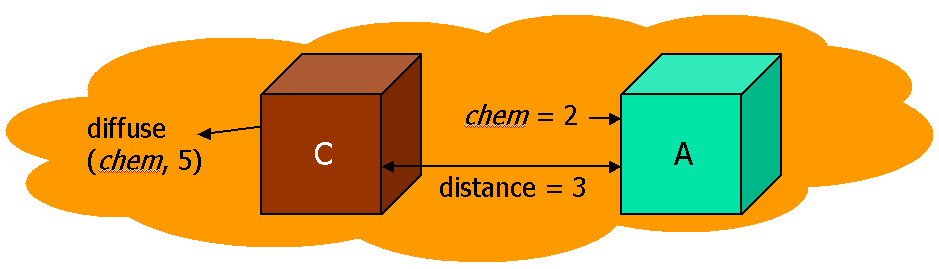
Cells can undergo a transition (i.e. state change or division) when they sense a diffused chemical just like any ordinary chemical

|
University of Virginia Department of Computer Science Programming the Swarm |
|
David Evans evans@cs.virginia.edu Selvin George selvin@cs.virginia.edu |